Case Reports
Share your love

Advantages of Neurogenic Stimulation and Identifying Ideal Dry Eye Candidates for Acoltremon | Ophthalmology Times
The Viewpoints series “Reimagining Dry Eye Management: Targeting Tear Function for Sustained Relief” convenes leading experts in ophthalmology and optometry to discuss evolving perspectives on dry eye disease (DED). Under the moderation of Kendall Donaldson, MD, MS, the panel—comprising Cecelia…

The Role of TRPM8 Agonists in Dry Eye Disease | Ophthalmology Times
The Viewpoints series “Reimagining Dry Eye Management: Targeting Tear Function for Sustained Relief” convenes leading experts in ophthalmology and optometry to discuss evolving perspectives on dry eye disease (DED). Under the moderation of Kendall Donaldson, MD, MS, the panel—comprising Cecelia…

Inflammasome Therapeutics completes phase 2 trial enrollment for K8 in GA | Ophthalmology Times
(Image Credit: AdobeStock) Inflammasome Therapeutics has completed enrollment in a multicenter phase 2 dose-ranging trial (NCT06164587) for its dual inflammasome inhibitor, K8, for geographic atrophy (GA). K8 is a member of a new class of inflammasome-inhibiting drugs called Kamuvudines. They…

Phase 1 trial confirms safety and tolerability of Huons’ dry eye therapy | Ophthalmology Times
(Image Credit: AdobeStock) Huons Co, a pharmaceutical subsidiary of Huons Group, received phase 1 Clinical Study Results (CSR) for HUC1-394, an eye drop for the treatment of dry eye disease (DED). HUC1-394 is a peptide-based eye drop for dry eyes,…

Kiora Pharmaceuticals receives patent for additional and novel formulations of KIO-100 compounds | Ophthalmology Times
(Image Credit: AdobeStock/ronstik) The US Patent and Trademark Office has issued a new patent (US Patent No. 12,472,263) on additional and novel formulations of the KIO-100 family of compounds from Kiora Pharmaceuticals. In July 2025, the company announced it had…
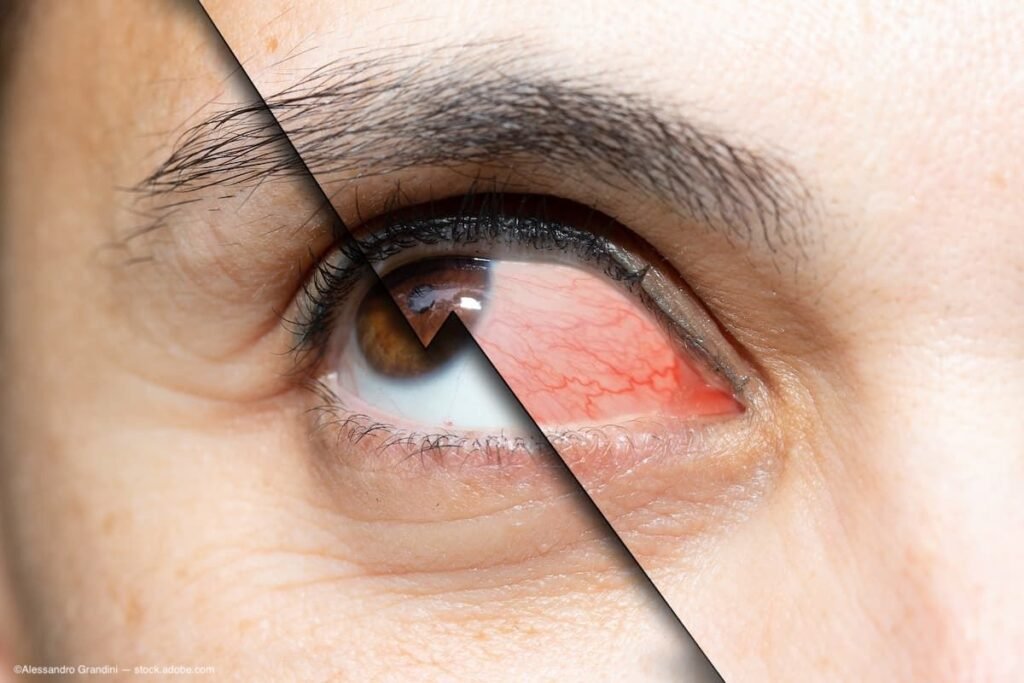
Collarettes common but largely stable in moderate-to-severe dry eye patients | Ophthalmology Times
(Image Credit: AdobeStock/Alessandro Grandini) In utilizing the Dry Eye Assessment and Management (DREAM) study data, a team of researchers found that lid margin collarettes were common in patients with moderate-to-severe dry eye disease (DED), but a majority of those patients’…

Clinical Experience and Integration of New Dry Eye Disease Therapies in the Clinic | Ophthalmology Times
The Viewpoints series “Reimagining Dry Eye Management: Targeting Tear Function for Sustained Relief” convenes leading experts in ophthalmology and optometry to discuss evolving perspectives on dry eye disease (DED). Under the moderation of Kendall Donaldson, MD, MS, the panel—comprising Cecelia…

Managing Unique Dry Eye Disease Subtypes in Patients | Ophthalmology Times
The Viewpoints series “Reimagining Dry Eye Management: Targeting Tear Function for Sustained Relief” convenes leading experts in ophthalmology and optometry to discuss evolving perspectives on dry eye disease (DED). Under the moderation of Kendall Donaldson, MD, MS, the panel—comprising Cecelia…

AGC Biologics to manufacture AAVantgarde’s dual-vector gene therapies, AVB-039 and AAVB-081 | Ophthalmology Times
(Image Credit: AdobeStock/Who is Danny) AAVantgarde and AGC Biologics have entered into an agreement in which AGC Biologics will provide Good Manufacturing Practice manufacturing for AAVantgarde’s dual-vector gene therapies for inherited retinal disorders, AAVB-039 and AAVB-081.1 Most recently, AAVantgarde Bio…
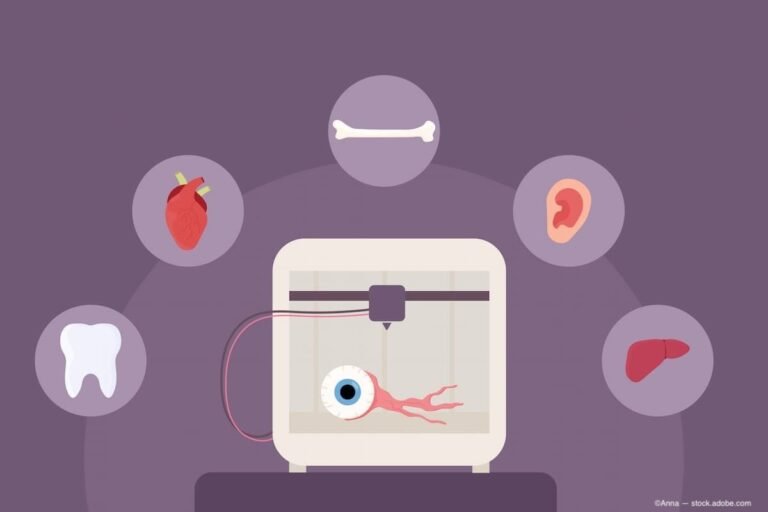
Precise Bio completes procedure using PB-001, a 3D-bioprinted corneal implant | Ophthalmology Times
(Image Credit: AdobeStock/Anna) Precise Bio has successfully treated a patient with PB-001, the company’s 3D-bio-printed corneal implant, at Rambam Medical Center in Haifa, Israel. The procedure was conducted as part of the company’s ongoing phase 1 clinical trial. PB-001 is…