
Newsletter Subscribe
Enter your email address below and subscribe to our newsletter


Enter your email address below and subscribe to our newsletter
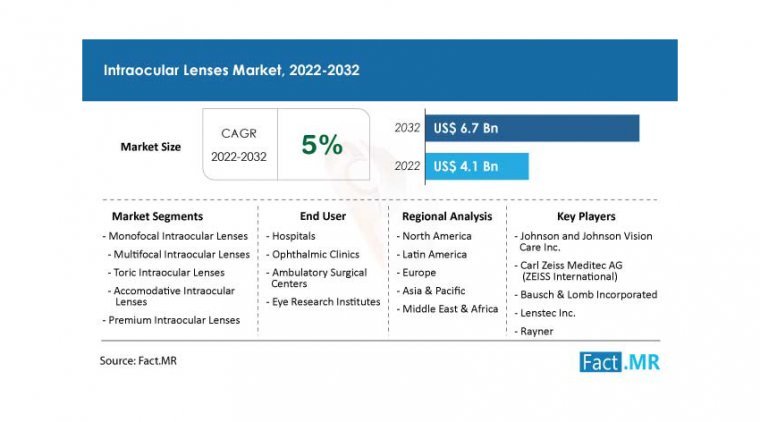
The global intraocular lens market is projected to grow from its current valuation of $4.1 billion to $6.7 billion by the end of 2032, according to a recent report by Fact Market Research (FMR). The market is expected to expand…
Regeneron Pharmaceuticals Inc. has won a patent case against Novartis AG in a U.S. court, protecting the company’s drug delivery system for its eye drug Eylea. The US Patent Trial and Appeal Board ruled in favor of Regeneron, stating that…

Contract research organization TFS HealthScience announced it has acquired Appletree CI Group, a CRO and global regulatory affairs service provider. The terms of the deal were not disclosed. The deal expands the global reach for clients while enhancing TFS HealthScience’s…

When Alexander Graham Bell brought us the revolutionary first ever telephone back in 1876, the world was changed forever. Ever since then, telephonic devices have been a mainstay of the modern world; an essential part of our everyday lives. Of…

Viatris announced the completion of its acquisitions of Oyster Point Pharma and Famy Life Sciences to establish a new Viatris Eye Care Division. On November 7, the purchase was originally announced. Jeffrey Nau, PhD, MMS, former CEO of Oyster Point…

Italy-based Sifi formed a collaboration with Avanzanite Bioscience, a Dutch specialty pharmaceutical business, to commercialize and distribute Akantior (polihexanide) exclusively in 26 European Economic Area and Swiss countries. Sifi will manage all ongoing regulatory activities under the terms of the…

Harrow announced the availability of Fortisite (compounded Tobramycin 1.5% + Vancomycin 5%) for in-office use from its FDA-registered and FDA-inspected ImprimisRx 503B outsourcing facility. Fortisite formulations are high-concentration, refrigeration-stable, fortified antibiotic formulations in solution that are patent-pending. According to Harrow,…
The FDA has approved Genentech’s Vabysmo (faricimab-svoa) for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME), marking the first bispecific antibody approved for the eye. Vabysmo is also the first and only FDA-approved injectable eye…

Amgen announced it is acquiring rare disease drug company Horizon Therapeutics, maker of eye disease drugs Tepezza and Uplizna, in a deal valued at $27.8 billion. The company will pay $116.50 in cash per share, a premium of 19.7% to…

Since producing the first IOL in the world in 1949, Rayner has led the way in innovation, focusing on giving you and your patients the best IOLs and opthalmic solutions while always being guided by research to enhance patient outcomes…