
Newsletter Subscribe
Enter your email address below and subscribe to our newsletter


Enter your email address below and subscribe to our newsletter

According to a recent systematic review and meta-analysis published online on Feb. 16 in JAMA Ophthalmology, the Amsler grid has a 67 percent sensitivity for detecting neovascular age-related macular degeneration (AMD) when compared with healthy controls. In a systematic literature…
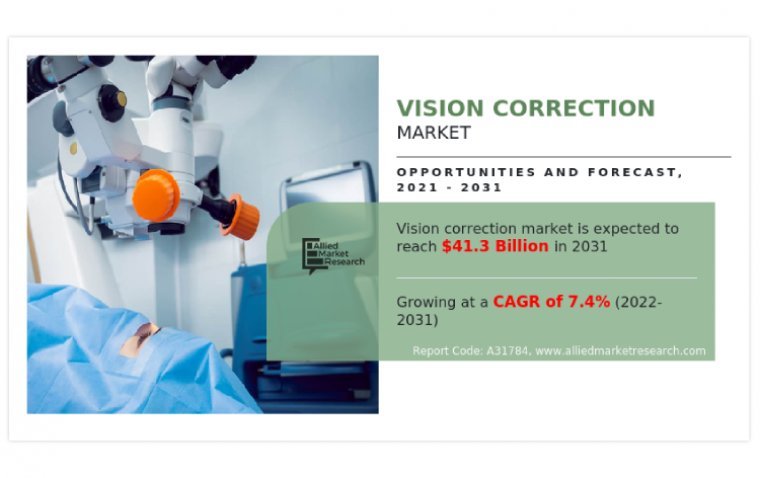
According to recent market research by Allied Market Research, the global vision correction market was worth $20,302.8 million in 2021, and is expected to grow at a compound annual growth rate (CAGR) of 7.4% from 2022 to 2031, reaching $41,252.7…

The European Parliament voted in favor of delaying the implementation of the new Medical Device Regulation (MDR) and approved the European Commission’s plan, which was adopted in January. The plan involves extending the deadlines for compliance with the new certification…

Frontera Therapeutics has initiated a clinical trial for its gene therapy, FT-002, which is intended for the treatment of X-linked retinitis pigmentosa (XLRP). The first patient has already been given a dose of the gene therapy product. FT-002 is Frontera’s…

The FDA issued a Warning Letter1 on January 31, 2023, to RightEye, LLC, the manufacturer of the RightEye Vision System, for misbranding and adulteration. The RightEye Vision System is a Class II Nystagmograph medical device that has been cleared “recording,…

Alcon Inc., a global leader in eye care, has reached a settlement agreement with J&J Surgical Vision to resolve their legal proceedings regarding femtosecond laser-assisted cataract surgery devices, including Alcon’s LenSx® device. Alcon acquired the LenSx device as part of…

Complement Therapeutics has made an announcement about the enrollment of the first participant in the i-GAIN study, a research project aimed at gaining insight into geographic atrophy (GA) – a condition that results in irreversible blindness and is caused by…
Regeneron Pharmaceuticals announced that the FDA granted its approval for the use of EYLEA® (aflibercept) Injection as a treatment option for preterm infants suffering from retinopathy of prematurity (ROP). This marks the first time that EYLEA has received approval for…

Endogena Therapeutics announced that the US FDA has designated the investigation of its drug EA-2353 for the treatment of retinitis pigmentosa (RP) as a Fast Track development program. This process speeds up the availability of new drugs for serious conditions…

Nidek has introduced the Cube α ophthalmic surgical system, which is designed to incorporate new ultrasound technology for enhanced phacoemulsification. Nidek reports that the Cube α features Gyro torsional technology within its compact design. The torsional ultrasound oscillation enhances the…