
Newsletter Subscribe
Enter your email address below and subscribe to our newsletter


Enter your email address below and subscribe to our newsletter

Photo of Patricio Schlottmann at the 2025 EURETINA meeting in Paris, France Patricio Schlottmann, MD, a retina specialist from Buenos Aires, Argentina, discusses the AVONELLE-X extension study of faricimab, a treatment for wet age-related macular degeneration (AMD). The study, spanning…
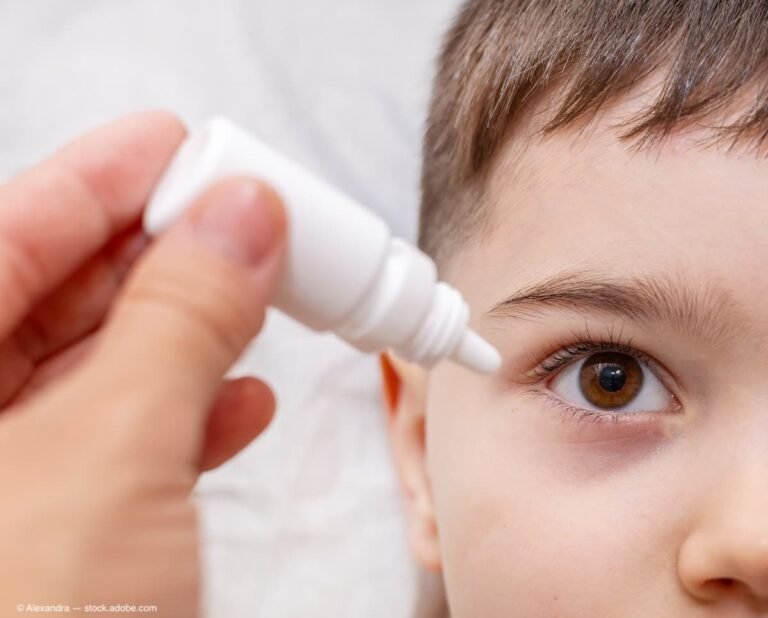
(Image Credit: AdobeStock) A comparison of 2 cycloplegic agents, atropine and cyclopentolate, in preschool children for cycloplegia found that atropine was associated with less myopic refraction compared with cyclopentolate and may potentially avoid the overestimation of premyopia prevalence,1 reported first…

(Image Credit: AdobeStock) Kala Bio announced that CHASE (NCT05727878), its phase 2b clinical trial of KPI-012 for persistent corneal epithelial defect (PCED), did not meet its primary endpoint. Additionally, the company noted that the trial failed to show statistical significance…

Photo of Marion Munk at EURETINA 2025 In her conversation with the Eye Care Network, Marion Munk, MD, PhD, details a promising phase 2a clinical study of ISTH0036, an oligonucleotide antisense drug targeting TGF-β2. Developed to address fibrosis in neovascular…

At this year’s EyeCon meeting, Oluwatosin U. Smith, MD, of the Glaucoma Associates of Texas, reflected on her dual role—both as an educator and a leader in its development—while delivering a focused presentation on the current state of glaucoma care.…

(Image Credit: AdobeStock) Canadian medical device company MacuMira Medical Devices recently introduced the first Health Canada-approved treatment to improve visual function for patients with dry age-related macular degeneration (AMD).1 This new technology, the MacuMira system, is a non-invasive treatment now…

(Image Credit: AdobeStock) The European Commission (EC) has granted marketing authorization for EYLUXVI (ALT-L9), an aflibercept (Eylea) biosimilar from Alteogen, co-developed by its subsidiary, Alteogen Biologics.1 EYLUXVI is Alteogen’s second approved biosimilar; the other approval is the company’s trastuzumab (Herceptin) biosimilar. Alteogen received a…

(Image credit: ©Alex_Traksel/AdobeStock) Luxa Biotechnology LLC announced results from its phase 1/2a clinical trial titled “Safety and Tolerability of RPE Stem Cell-derived RPE (RPESC-RPE) Transplantation in Patients with Dry Age-related Macular Degeneration: Low Dose Clinical Outcomes.”1 The results were published…

OKYO Pharma has announced its plans for the next stage of clinical development of urcosimod (formerly OK-101), its lead drug candidate to treat neuropathic corneal pain (NCP). NCP is a condition that causes severe pain and sensitivity of the eyes, face,…

Note: Captions are generated with the assistance of AI. Justis Ehlers, MD, spoke with Ophthalmology Times to share key findings from his recent presentation at the 2025 Retina Society meeting, which was held in Chicago, Illinois. Ehlers shared data from…