Innovation
Share your love

The potential of AI in retina with Daniela Ferrara
This conversation features Daniella Ferrara, Chief Medical Officer for TopCon Healthcare, discussing the transformative potential of artificial intelligence in retinal imaging and ophthalmology. Ferrara, an ophthalmologist and retina specialist with 25 years of experience in retinal imaging innovation. The discussion…

VivaVision Biotech appoints Quan Dong Nguyen to its scientific advisory board
(Image credit: AdobeStock) VivaVision Biotech Co., Ltd., appointed Quan Dong Nguyen, MD, MSc, FAAO, FARVO, FASRS, to its scientific advisory board. Nguyen is a professor of ophthalmology at the Byers Eye Institute, Stanford University School of Medicine. He is also…

AEVR’s executive director on #SeeWhatMatters and safeguarding the National Eye Institute
#SeeWhatMatters is a national advocacy campaign to raise awareness about the importance of federally funded vision research and to protect the independence of the National Eye Institute amid proposed structural changes to the NIH. (Image courtesy of #SeeWhatMatters) The Alliance…
JP Morgan banker says innovation will drive ophthalmology through current market cycle – Healio
JP Morgan banker says innovation will drive ophthalmology through current market cycle Healio Source: Author: | Date: 2025-04-27 07:00:00 Source: Author: | Date: 2025-04-27 07:00:00
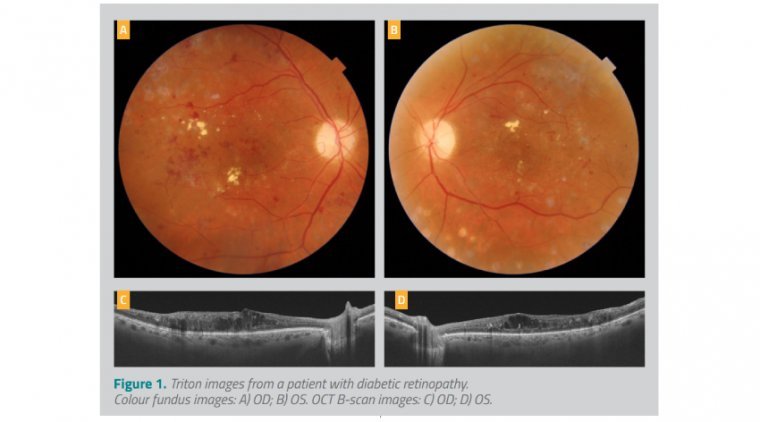
What is optical coherence tomography
Optical coherence tomography (OCT) has been a cornerstone tool for many years for the diagnosis and management of conditions involving the posterior segment. Although continuous hardware and software advances have increased the applications and utility of OCT, multimodal imaging that…

A Next-Gen Monofocal IOL From Johnson & Johnson Vision
Johnson & Johnson Vision Announces Launch of TECNIS Eyhance™ Toric II IOL with TECNIS Simplicity™ Delivery System, a Next-Generation Monofocal Intraocular Lens for Cataracts Patients with Astigmatism, in Europe TECNIS Eyhance™ Toric II IOL offers all the benefits associated with…

J&J announced new products at the AAO annual meeting
Johnson & Johnson Vision announced a series of new products and innovations at the 2019 American Academy of Ophthalmology (AAO) Annual Meeting in San Francisco, CA, USA. The company announced the US Food & Drug Administration (FDA) approval of the…

VSY Biotech Signs Agreement With WAKAMOTO Pharma
Leinfelden – Echterdingen, Germany March 19, 2021, VSY Biotechnology GmbH, an innovation-driven company with its progressive R&D activities in ophthalmology, visco supplementation in orthopedy and medical esthetics, announced today an agreement with WAKAMOTO PHARMACEUTICAL CO., LTD. (hereinafter refer to…

HealthCare Royalty And Clearside Bio to Enter Into Agreement
Clearside Biomedical announced it has entered into an agreement with HealthCare Royalty Partners in a deal worth up to $65 million. The money received from the deal will be put to use by Clearside to fund the ongoing clinical testing…

Rayner’s RayOne EMV IOL Approved By FDA I OBN
Since producing the first IOL in the world in 1949, Rayner has led the way in innovation, focusing on giving you and your patients the best IOLs and opthalmic solutions while always being guided by research to enhance patient outcomes…