
Newsletter Subscribe
Enter your email address below and subscribe to our newsletter


Enter your email address below and subscribe to our newsletter
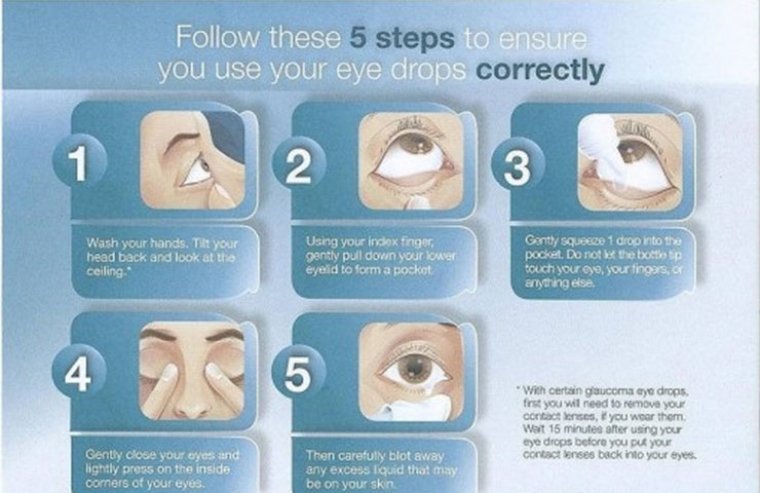
When patients are diagnosed with glaucoma, one of the most common treatment options is a prescription eye drop medication that lowers eye pressure, which prevents damage to the optic nerve and helps to preserve vision. Several types of drugs lower…

Johnson & Johnson Vision announced a series of new products and innovations at the 2019 American Academy of Ophthalmology (AAO) Annual Meeting in San Francisco, CA, USA. The company announced the US Food & Drug Administration (FDA) approval of the…
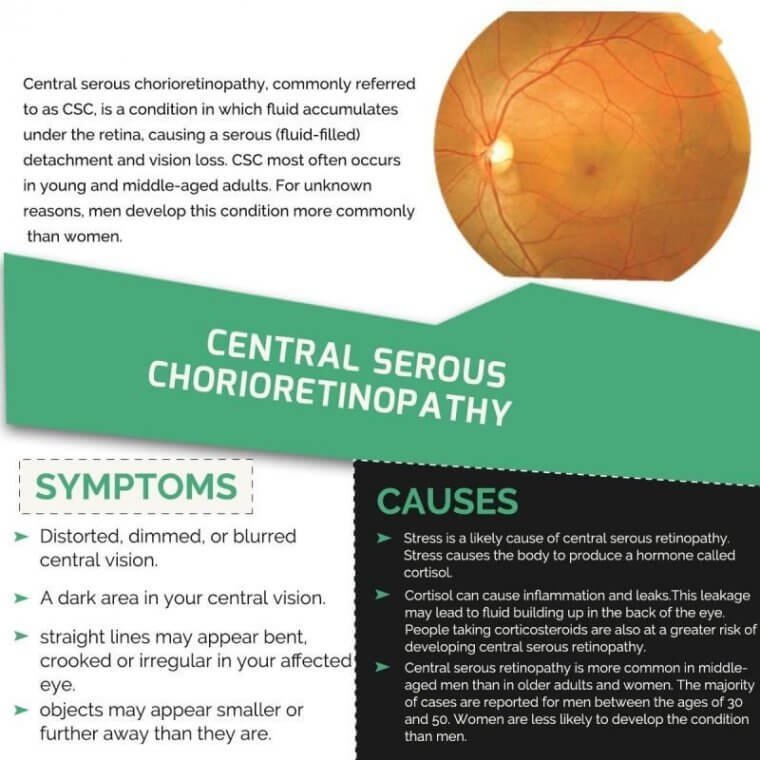
Central serous chorioretinopathy, commonly referred to as CSC, is a condition in which fluid accumulates under the retina, causing a serous (fluid-filled) detachment and vision loss. CSC most often occurs in young and middle-aged adults. For unknown reasons, men develop…

Topcon Healthcare Solutions and OphtAI, a joint venture specialized in Artificial Intelligence (AI) for ophthalmology, have announced a partnership to integrate OphtAI into Topcon’s Harmony Referral System, and provide eye care professionals with smarter access to AI services. OphtAI’s state-of-the-art…

Johnson & Johnson Vision has announced that they have received CE Mark approval for the ELITA Femtosecond Laser System. The laser is designed to perform refractive correction on patients with myopia, with or without astigmatism, using the SILK (Smooth Incision…

According to a recent systematic review and meta-analysis published online on Feb. 16 in JAMA Ophthalmology, the Amsler grid has a 67 percent sensitivity for detecting neovascular age-related macular degeneration (AMD) when compared with healthy controls. In a systematic literature…

Frontera Therapeutics has initiated a clinical trial for its gene therapy, FT-002, which is intended for the treatment of X-linked retinitis pigmentosa (XLRP). The first patient has already been given a dose of the gene therapy product. FT-002 is Frontera’s…

Complement Therapeutics has made an announcement about the enrollment of the first participant in the i-GAIN study, a research project aimed at gaining insight into geographic atrophy (GA) – a condition that results in irreversible blindness and is caused by…
Regeneron Pharmaceuticals announced that the FDA granted its approval for the use of EYLEA® (aflibercept) Injection as a treatment option for preterm infants suffering from retinopathy of prematurity (ROP). This marks the first time that EYLEA has received approval for…

Endogena Therapeutics announced that the US FDA has designated the investigation of its drug EA-2353 for the treatment of retinitis pigmentosa (RP) as a Fast Track development program. This process speeds up the availability of new drugs for serious conditions…