Patient Education
Share your love

Nidek Introduces Cube Alpha Ophthalmic Surgical System
Nidek has introduced the Cube α ophthalmic surgical system, which is designed to incorporate new ultrasound technology for enhanced phacoemulsification. Nidek reports that the Cube α features Gyro torsional technology within its compact design. The torsional ultrasound oscillation enhances the…

Nidek and Hoya Form Global Partnership
Hoya Vision Care and Nidek have entered a global partnership that would give Hoya’s clients access to Nidek’s optical goods, services, and equipment through both regional distributors. Financial terms of the deal were not disclosed. According to Nidek, the partnership’s…
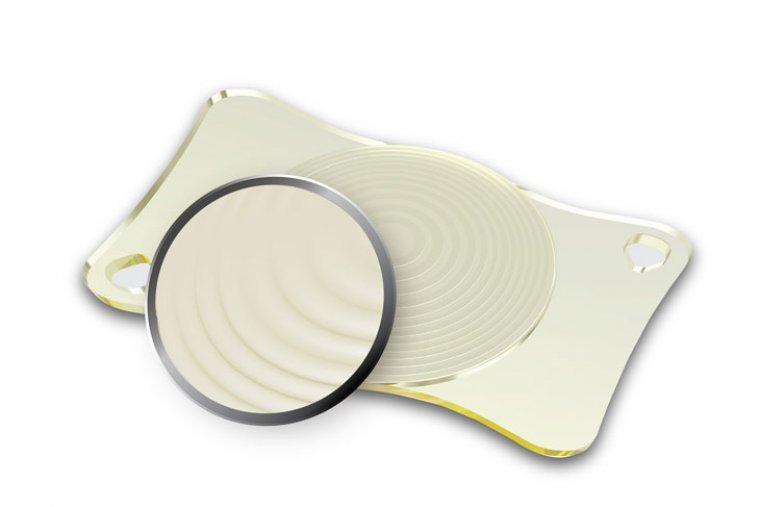
World’s First And Only Sinusoidal Trifocal Intra Ocular Lens
AcrivaUD Trinova Comes to the Fore with its Four Main Features The world’s first and only sinusoidal trifocal iol Acrivaud trinova used by ophthalmologists in 64 countries across the world to correct cataracts and for refractive lens exchange attracts more…

Ximluci Receives Marketing Approval in the UK
STADA Arzneimittel AG and Xbrane Biopharma AB announced that the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has approved Ximluci® (ranibizumab), a biosimilar of Lucentis®. STADA plans to launch Ximluci in the United Kingdom in 2023. Xbrane’s contribution to…

Sifi and Avanzanite Sign License Agreement
Italy-based Sifi formed a collaboration with Avanzanite Bioscience, a Dutch specialty pharmaceutical business, to commercialize and distribute Akantior (polihexanide) exclusively in 26 European Economic Area and Swiss countries. Sifi will manage all ongoing regulatory activities under the terms of the…

Harrow Now Offering Fortisite Formulations for In-Office Use
Harrow announced the availability of Fortisite (compounded Tobramycin 1.5% + Vancomycin 5%) for in-office use from its FDA-registered and FDA-inspected ImprimisRx 503B outsourcing facility. Fortisite formulations are high-concentration, refrigeration-stable, fortified antibiotic formulations in solution that are patent-pending. According to Harrow,…
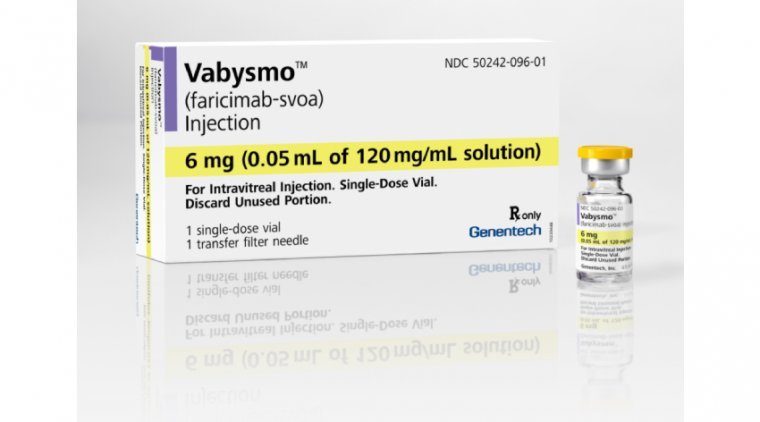
FDA Greenlights Genentech’s Vabysmo | OBN
The FDA has approved Genentech’s Vabysmo (faricimab-svoa) for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME), marking the first bispecific antibody approved for the eye. Vabysmo is also the first and only FDA-approved injectable eye…

Alcon’s New Eye Health Education and Training Center I OBN
Alcon has officially inaugurated the new Alcon Experience Center (AEC) in Barcelona, one of Europe’s largest eye health education and training facilities, providing eye care professionals with the chance to learn about and practice the latest advancements in ophthalmology and…

Rayner’s RayOne EMV IOL Approved By FDA I OBN
Since producing the first IOL in the world in 1949, Rayner has led the way in innovation, focusing on giving you and your patients the best IOLs and opthalmic solutions while always being guided by research to enhance patient outcomes…