Treatment
Share your love
Vizz officially launches in the United States | Ophthalmology Times
(Image Credit: AdobeStock) Following a US Food and Drug Administration approval back in July 2025, LENZ Therapeutics has announced that VIZZ (aceclidine ophthalmic solution) 1.44% is now commercially available.1 The first and only FDA-approved aceclidine-based eye drop is for the…

David Lally discusses the ARCHER trial and the optimistic outcomes fueling future research | Ophthalmology Times
Note: Video captions are generated with the assistance of AI and may contain errors. David Lally, MD, presented at the 2025 Retina Society meeting, discussing the ARCHER study (NCT04656561) of ANX007 for the treatment of age-related macular degeneration (AMD) and…

Lupin signs definitive agreement to acquire VISUfarma | Ophthalmology Times
(Image Credit: AdobeStock/Rido) Lupin’s subsidiary, Nanomi, has signed a definitive agreement to acquire VISUfarma, a portfolio company of GHO Capital Partners LLP. Lupin notes that the acquisition will help the company expand its European business, as well as advance the…

Positive pediatric data emerge from the OPGx-LCA5 phase 1/2 trial of Leber congenital amaurosis type 5 | Ophthalmology Times
Image credit: AdobeStock/arturkowynia Opus Genetics today announced the positive 3-month pediatric data from its Phase 1/2 open-label, ascending-dose study of the safety and efficacy of OPGx-LCA5, an investigational gene therapy for Leber congenital amaurosis type 5 (LCA5), an ultra-rare form…

Q&A: Patricio Schlottmann discusses results of AVONELLE-X faricimab extension trial | Ophthalmology Times
Photo of Patricio Schlottmann at the 2025 EURETINA meeting in Paris, France Patricio Schlottmann, MD, a retina specialist from Buenos Aires, Argentina, discusses the AVONELLE-X extension study of faricimab, a treatment for wet age-related macular degeneration (AMD). The study, spanning…

Q&A: Alexandra Miere discusses the ACTOR and HERMES studies | Ophthalmology Times
Photo of Alexandra Miere at the 2025 EURETINA meeting in Paris Alexandra Miere discusses retinal studies published in EURETINA journal. The first, the ACTOR study, explores vascular remodeling in neovascular AMD patients treated with ranibizumab. The second, the HERMES study,…
Researchers assess atropine and cyclopentolate for detecting premyopia in young children | Ophthalmology Times
(Image Credit: AdobeStock) A comparison of 2 cycloplegic agents, atropine and cyclopentolate, in preschool children for cycloplegia found that atropine was associated with less myopic refraction compared with cyclopentolate and may potentially avoid the overestimation of premyopia prevalence,1 reported first…
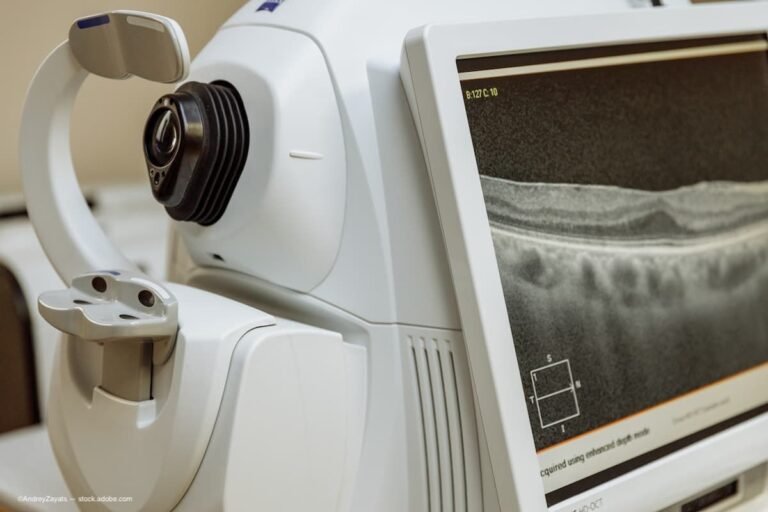
Anniversary of the spectral OCT prototype: 25 years young and growing | Ophthalmology Times
A recent conference celebrated the creation of the first prototype of the spectral optical coherence tomography (SOCT) instrument. Introduced in 2000, the prototype, which was developed at the Institute of Physics of Nicolaus Copernicus University in Toruń, Poland, was the…

Kala Bio’s CHASE trial misses primary endpoint, KPI-012 development discontinued | Ophthalmology Times
(Image Credit: AdobeStock) Kala Bio announced that CHASE (NCT05727878), its phase 2b clinical trial of KPI-012 for persistent corneal epithelial defect (PCED), did not meet its primary endpoint. Additionally, the company noted that the trial failed to show statistical significance…

Q&A: Mark Gillies the outcomes of injecting VEGF inhibitors | Ophthalmology Times
Photo of Mark Gillies at the 2025 EURETINA meeting Mark Gillies, MD, PhD, introduced the Fight Retinal Blindness! Project, a comprehensive registry tracking VEGF inhibitor eye injection outcomes since 2007. Spanning 20 countries with 150 active practitioners, the project uniquely…